AI-based 3D modelling for cancer immunotherapy design
Immunotherapy, particularly T cell–based therapies, has revolutionized cancer treatment, with some advanced-stage patients achieving complete tumor regression. By training the immune system to recognize and destroy cancer cells, immunotherapy represents a paradigm shift in oncology. However, its broader impact is constrained by several challenges: prohibitive costs (e.g., Kimmtrak at $18,760 per vial for a weekly dose), lengthy development timelines, treatment-related toxicities, and the fact that only a fraction of patients currently benefit.
A central question remains: How do T cells distinguish self from non-self, and which tumor mutations provide the most effective therapeutic targets? For a tumor-derived peptide to be a viable target, it must (1) bind strongly to the patient’s MHC proteins to be displayed on the cell surface, and (2) be recognized by T cell receptors (TCRs) as foreign. While many computational methods have been developed to predict these interactions, their accuracy remains limited. This limitation arises largely because most state-of-the-art methods rely on sequence-based models—approaches that are data-hungry and struggle to generalize to unseen cases.
The labs of Erik Bekkers (UvA) and Li Xue (Radboudumc) pioneer geometric deep learning (GDL) [1-4] and 3D structure–based approaches for cancer immunotherapy design [5–10]. Unlike 1D sequence data, 3D structures capture rich biophysical information, encoding the fundamental energetic and spatial rules that govern molecular recognition. As a result, structure-based models offer greater generalizability and data efficiency.
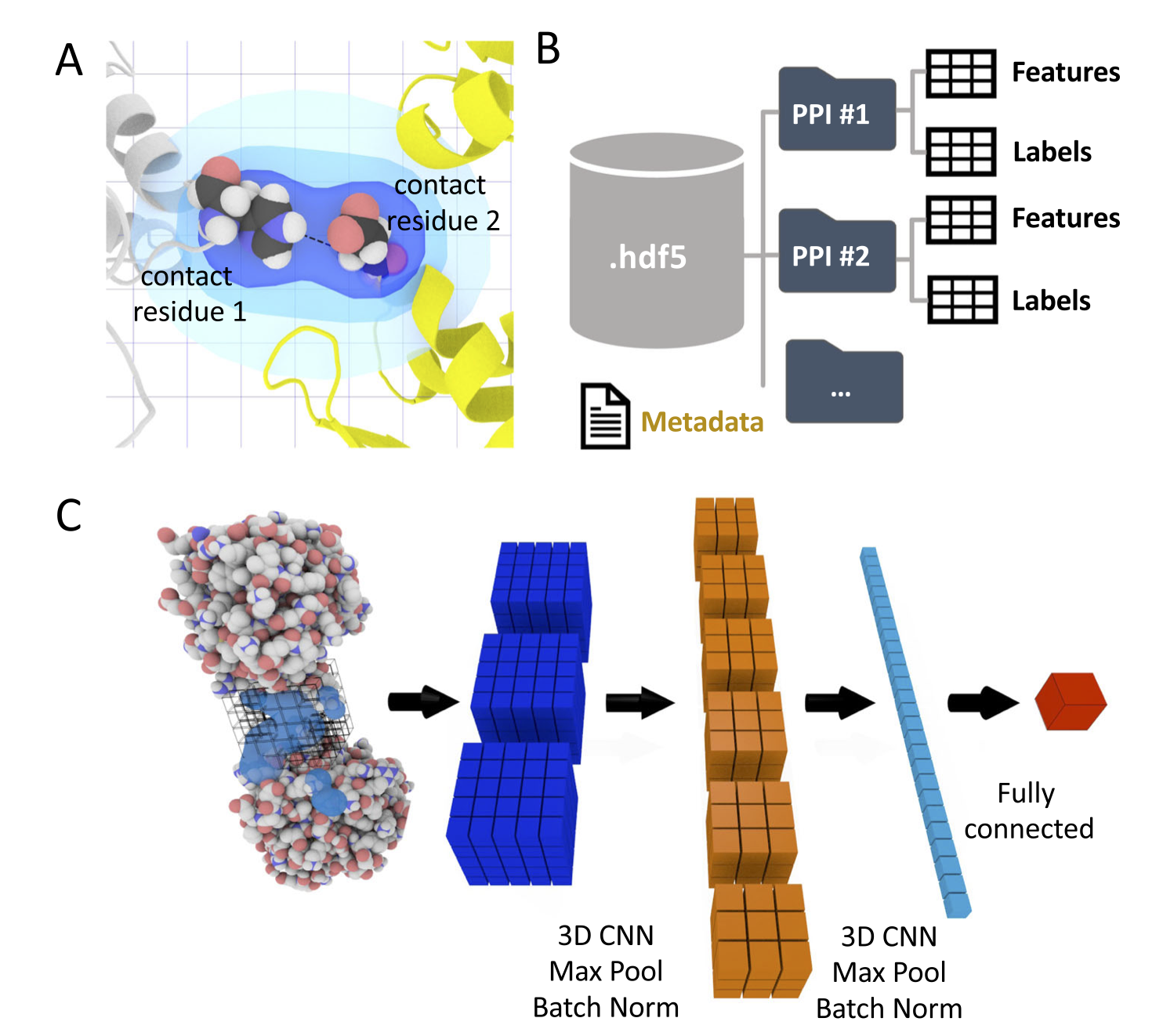 Figure 1: The DeepRank framework for 3D protein-protein interfaces [5].
Figure 1: The DeepRank framework for 3D protein-protein interfaces [5].
In our recent work [8], we demonstrated that 3D structure–based methods outperform sequence-based approaches in predictive power and transferability. Extending natural language processing (NLP) innovations (e.g., ChatGPT) into 3D, we developed a novel 3D self-supervised learning (3D-SSL) framework, achieving superior data efficiency [8]. We further showed that task-specific small AI models can be up to 1000× faster while matching or surpassing the performance of large, general-purpose models such as AlphaFold [5]. In addition, we designed an equivariant graph neural network (EGNN)–based diffusion models for accurate 3D modeling of peptide–MHC complexes [11].
We are actively expanding this research frontier and welcome talented students to join us in driving the next breakthroughs in computational immunotherapy. Our collaboration with the Bekkers GDL lab has already led to four successfully supervised master theses, one publication, and another currently in submission. Explore our latest international symposium here: https://x-lab-3d.github.io/csi/.
Our open projects:
- Project 1: Flow Matching for 3D Peptide–MHC Modeling and binding predictions
- Project 2: Efficient energy guided sampling of realistic protein conformations via latent space representations
- Project 3: Structure-Based Immunogenicity Prediction through E(n) equivariant networks
Each project is intended as MSc thesis project. There may be a possibility for funding through AI4Health at Radboudumc, although the application process is competitive, and not guaranteed.
Contact: Li Xue: (li.xue@radboudumc.nl), Erik Bekkers: (e.j.bekkers@uva.nl), or Tom Claassen (Tom.Claassen@ru.nl)
References
- Bekkers, E. J.; Vadgama, S.; Hesselink, R.; Linden, P. A. V. der; Romero, D. W. Fast, Expressive SE(n) Equivariant Networks through Weight-Sharing in Position-Orientation Space; 2023.
- Vadgama, S.; Islam, M. M.; Buracas, D.; Shewmake, C.; Moskalev, A.; Bekkers, E. Probing Equivariance and Symmetry Breaking in Convolutional Networks. arXiv June 2, 2025. https://doi.org/10.48550/arXiv.2501.01999.
- Islam, M.; …, Bekkers, E.J. The Platonic Transformer: Unifying Group Convolution and Linear-Time Attention. In review at ICLR 2026.
- Zaghen, O.; Eijkelboom, F.; Pouplin, A.; Bekkers, E.J. Riemannian Variational Flow Matching for Material and Protein Design. In review at ICLR 2026
- Renaud, N.; Geng, C.; Georgievska, S.; Ambrosetti, F.; Ridder, L.; Marzella, D. F.; Réau, M. F.; Bonvin, A. M. J. J.; Xue, L. C. DeepRank: A Deep Learning Framework for Data Mining 3D Protein-Protein Interfaces. Nat. Commun. 2021, 12 (1), 7068. https://doi.org/10.1038/s41467-021-27396-0.
- Crocioni, G.; Bodor, D. L.; Baakman, C.; Parizi, F. M.; Rademaker, D.-T.; Ramakrishnan, G.; Burg, S. A. van der; Marzella, D. F.; Teixeira, J. M. c; Xue, L. C. DeepRank2: Mining 3D Protein Structures with Geometric Deep Learning. J. Open Source Softw. 2024, 9 (94), 5983. https://doi.org/10.21105/joss.05983.
- Baakman, C.; Crocioni, G.; Rademaker, D. T.; Frühbuß, D.; Aarts, Y. J. M.; Xue, L. C. SwiftMHC: A High-Speed Attention Network for MHC-Bound Peptide Identification and 3D Modeling. bioRxiv January 22, 2025, p 2025.01.20.633893. https://doi.org/10.1101/2025.01.20.633893.
- Marzella, D. F.; Crocioni, G.; Radusinovic, T.; Lepikhov, D.; Severin, H.; Bodor, D. L.; Rademaker, D. T.; Lin, C.; Georgievska, S.; Kessler, A. L.; Lopez-Tarifa, P.; Buschow, S.; Bekkers, E.; Xue, L. C. Improving Generalizability for MHC-Binding Peptide Predictions through Structure-Based Geometric Deep Learning. bioRxiv December 5, 2023, p 2023.12.04.569776. https://doi.org/10.1101/2023.12.04.569776.
- Marzella, D. F.; Parizi, F. M.; Tilborg, D. V.; Renaud, N.; Sybrandi, D.; Buzatu, R.; Rademaker, D. T.; ‘T Hoen, P. A. C.; Xue, L. C. PANDORA: A Fast, Anchor-Restrained Modelling Protocol for Peptide: MHC Complexes. Front. Immunol. 2022, 13, 878762. https://doi.org/zd.
- Parizi, F. M.; Aarts, Y. J. M.; Eerden, S.; Ramakrishnan, G.; Xue, L. C. SwiftTCR: Efficient Computational Docking Protocol of TCRpMHC-I Complexes Using Restricted Rotation Matrices. bioRxiv 2024. https://doi.org/10.1101/2024.05.27.596020.
- Frühbuß, D.; Baakman, C.; Teusink, S.; Bekkers, E.; Jegelka, S.; Xue, L. C. Fast and Accurate Peptide–MHC Structure Prediction via an Equivariant Diffusion Model. bioRxiv May 1, 2025, p 2025.04.28.650973. https://doi.org/10.1101/2025.04.28.650973.
